Isolate Quiz Winner
Thank you to everyone who tried their hand at the isolates quiz this week! You all did a pretty good research job - I'm impressed :-)
Here are the questions again - plus the correct answers:
1) What is the name of the molecule that gives spearmint its characteristic scent?
Spearmint has many components in it, but the most characteristic is l-carvone. The "l" is short for laevo-carvone, or levorotary, which means "left handed" - as the propyl radical C3H5 points to the left. In d-carvone, the right-handed (dextorotary) propyl radical creates the characteristic scent of dill or is a little similar to caraway. The formula for both molecules is C10H14O, but their structure is different, resulting in two very distinctive smells.
2) What's the common isolate for these three oils: Hay Lime and Tonka Bean?
Coumarin. Yes, lime is an unusual citrus in that it contains coumarin!
3) What isolate is used to produce the drug Ecstasy?
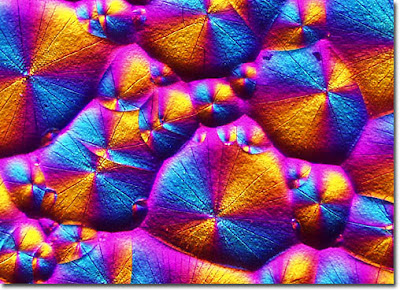
No one got this one. You all guessed safrole, which is often the start material for heliotropin - which is the correct answer for this question. Also known as piperonal (whose micromagnetic photo was used to illustrate that post), heliotropin requires special licensing when purchased in the USA. It smells like almonds and vanilla, or cherry pie, and very similar to the beautiful scent of the flower heliotrope. More about the flower at another time!
4) What's a characteristic molecule that's common to orange blossom, tuberose and ylang ylang?
Methyl anthranilate. This ester smells like concord grapes with hints of wintergreen, and is responsible for the sweet-fruity, slightly medicinal aspect of these essences. It's also present in jasmine, but I did not want to mention jasmine as to not confuse you with indole. Indole, by the way, is not present in ylang ylang or tuberose. What gives ylang ylang its characteristic animal scent is another molecule altogether, called paracresyl methyl ether.
5) What does citral smell like? And what plant(s) essential oil(s) has/have the highest citral content?
I made this one too easy for you. I was going to select Geraniol, but knew it would be too confusing. So yes, most of you got this correctly. Citral is a characteristic smell of lemon, although most of lemon is just limonene (generic lemon-orange scent). It's the citral that gives it the sweet "lemon drop" aspect, which is even furthermore pronounced in Litsea Cubeba and Lemon Myrtle. It's also present in large amounts in citronella, lemon eucalyptus, lemongrass, lemon verbena, lemon balms and others - but it is very easily recognizable and dominant in Litsea Cubeba aka May Chang, and in Lemon Myrtle, where it comprises more than 90% of the oil.
Citral is of significance, because it is the starting point of many other natural compounds that would have been very expensive to produce or isolate directly from the plant. For example: alpha ionone, which smells nothing like citral - but has the warm-woody scent of candied violets!
Because many of those who answered correctly have already won more than once on SmellyBlog, the winner was not randomly selected this time, but hand-picked by the editor. Congratulations to
Here are the questions again - plus the correct answers:
1) What is the name of the molecule that gives spearmint its characteristic scent?
Spearmint has many components in it, but the most characteristic is l-carvone. The "l" is short for laevo-carvone, or levorotary, which means "left handed" - as the propyl radical C3H5 points to the left. In d-carvone, the right-handed (dextorotary) propyl radical creates the characteristic scent of dill or is a little similar to caraway. The formula for both molecules is C10H14O, but their structure is different, resulting in two very distinctive smells.
2) What's the common isolate for these three oils: Hay Lime and Tonka Bean?
Coumarin. Yes, lime is an unusual citrus in that it contains coumarin!
3) What isolate is used to produce the drug Ecstasy?
No one got this one. You all guessed safrole, which is often the start material for heliotropin - which is the correct answer for this question. Also known as piperonal (whose micromagnetic photo was used to illustrate that post), heliotropin requires special licensing when purchased in the USA. It smells like almonds and vanilla, or cherry pie, and very similar to the beautiful scent of the flower heliotrope. More about the flower at another time!
4) What's a characteristic molecule that's common to orange blossom, tuberose and ylang ylang?
Methyl anthranilate. This ester smells like concord grapes with hints of wintergreen, and is responsible for the sweet-fruity, slightly medicinal aspect of these essences. It's also present in jasmine, but I did not want to mention jasmine as to not confuse you with indole. Indole, by the way, is not present in ylang ylang or tuberose. What gives ylang ylang its characteristic animal scent is another molecule altogether, called paracresyl methyl ether.
5) What does citral smell like? And what plant(s) essential oil(s) has/have the highest citral content?
I made this one too easy for you. I was going to select Geraniol, but knew it would be too confusing. So yes, most of you got this correctly. Citral is a characteristic smell of lemon, although most of lemon is just limonene (generic lemon-orange scent). It's the citral that gives it the sweet "lemon drop" aspect, which is even furthermore pronounced in Litsea Cubeba and Lemon Myrtle. It's also present in large amounts in citronella, lemon eucalyptus, lemongrass, lemon verbena, lemon balms and others - but it is very easily recognizable and dominant in Litsea Cubeba aka May Chang, and in Lemon Myrtle, where it comprises more than 90% of the oil.
Citral is of significance, because it is the starting point of many other natural compounds that would have been very expensive to produce or isolate directly from the plant. For example: alpha ionone, which smells nothing like citral - but has the warm-woody scent of candied violets!
Because many of those who answered correctly have already won more than once on SmellyBlog, the winner was not randomly selected this time, but hand-picked by the editor. Congratulations to